|
Nitreg®
Potential-Controlled Gas Nitriding
- Nitreg® -氮势 – 可控氮化
亲爱的访问者,
在您探访本站之前, 请注意该部分网页内容正在建造中.
对由此引起您的不便我们真诚表达歉意.
请在英文网页继续您的浏览. 如果无法找到您需要的信息请与我们联系.
What is nitriding?
Before a specific explanation of Nitreg® can be effectively presented, as well
as for the sake of completeness, we will describe nitriding in general. Then we
will proceed to describe our “potential-control” group of technologies.
Nitriding is a process of diffusing nitrogen atoms into the metal’s surface.
Nitrogen is plentiful on Earth, however, in nature it exists as a two-atom
molecule, chemically inert and too large to penetrate the surface. Hence
nitriding technologies focus on the source of nascent (atomic) nitrogen.
Regardless of the method, nitriding is
a process of diffusing nitrogen into the metal and such diffusion, once
individual atoms of nitrogen have penetrated the surface, continues as long as
the temperature is high enough, and there is a fresh supply of nascent nitrogen
on the surface. In other words, the diffusion is basically the same in all
nitriding, while the difference lies in the supply of nitrogen. The latter has a
fundamental influence on the resultant properties of the surface.
Traditional Nitriding Methods
The three traditional nitriding
methods practiced on an industrial scale are:
(a) salt bath (liquid) nitriding,
where the source of nitrogen (and also carbon) is molten salt,
(b) gas nitriding which uses ammonia
(NH3), and
(c) plasma nitriding where molecular
nitrogen (N2) is split into ions in an electromagnetic field.
Salt baths are not used by Nitrex due to that method’s environmental dangers and
our concern for personal safety of the operators. Besides, the process has few
advantages, quick heating of the workpiece being the only one worth mentioning.
Therefore the process shall not be described any further.
Plasma nitriding is used by Nitrex for selected applications and the process is
described as a
separate paragraph in the “Technology” section.
Nascent nitrogen is obtained from ammonia in gas nitriding. The conventional
version of the process relies on the measurement of the dissociation rate of
ammonia into its constituent gases – nitrogen and hydrogen. A simple device
called a buret (or more properly burette) is employed for periodically checking
the dissociation rate and an adjustment to the flow of ammonia is made as
required, usually in a manual fashion.
Materials
Generally speaking all ferrous alloys,
including stainless steels, cast irons, and even titanium alloys, are capable of
being nitrided. However, the various alloys have different characteristics with
regard to surface conditions, the natural speed of diffusion and propensity to
form nitrides. It is important to understand that even a properly run nitriding
process will produce significantly different results on dissimilar materials.
Consequently, some users may have insurmountable difficulties, particularly if
their methodology is primitive and/or their knowledge and experience inadequate.
Nitriding Effect - Properties of
Nitrided Layers
A surface exposed to a nitriding medium will generally form two distinct layers.
The outside layer is called a compound layer (or white layer) and its thickness
generally falls between zero and 0.001” (25
mm). Underneath the white layer we have a diffusion case or diffusion
zone. Both together comprise what is generally referred to as the case. However,
as mentioned earlier, depending on the material and its original pre-process
hardness there will be very significant differences between the properties of
these layers. A full description of these phenomena is well outside the scope of
this website. We encourage our visitors to contact us if specific such
information is required. Meanwhile we will concentrate our attention on
explaining the “why and how” of controlling the properties of the nitrided
surface, particularly with regard to the compound layer.
Control
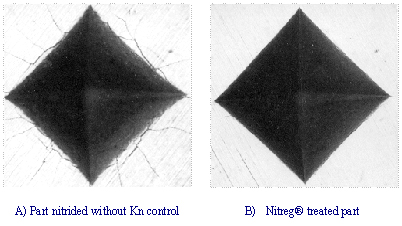
The images of two Vickers hardness
tester indentations shown below illustrate the difference between a controlled
and uncontrolled process. The specimen on the left was produced in a traditional
process and the cracking of the surface is indicative of the brittleness of the
layer. The one on the right is a product of a Nitreg® process where, in spite of
the same hardness, cracks have not formed. The Nitreg® treated component is
therefore more resilient with high toughness of the compound layer.
Such superior result can only be
achieved by controlling the nitrogen concentration in the substrate and the
modern approach is control of nitriding potential (Kn). Proper understanding and
application of the principles that tie nitriding potential (Kn), temperature and
time are the cornerstone of the Nitreg® technology. An example of our ability to
produce a variety of white layer / diffusion case combinations is shown in the
following chart:
Nitrided case combinations -
Acrobat®
Gradually the ability to control the
nitriding potential is becoming a requirement as set forth by specifications
such as AMS 2759/10.
In
conclusion, Nitreg® is a modern process, capable of meeting the metallurgical
requirements of all nitriding specifications that may have been originally
written for salt bath, plasma or traditional gas nitriding
|